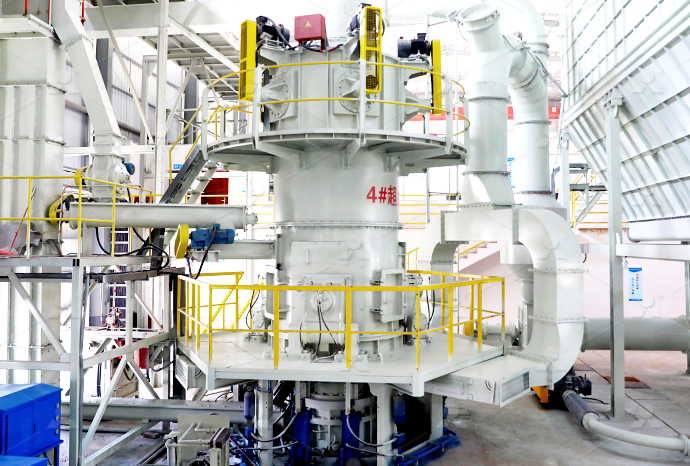
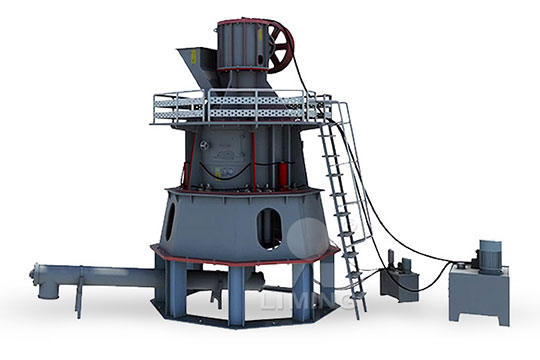
Advantages of continuous quicklime calcium carbonate

Natural and enhanced carbonation of lime in its
2021年10月4日 Lime is a product derived from the thermal decomposition of limestone (mainly calcium carbonate, CaCO 3) into quicklime (CaO) and carbon dioxide (CO 2), also called calcination Controlled reaction with water is used 2021年1月1日 It allows permanent CO2 storage in a stable product since the lime combines with gaseous CO2 reforming CaCO3 This paper reports a comprehensive literature review on the carbonation potential of(PDF) Natural and enhanced carbonation of lime 2023年8月22日 the steel slag is the consequence of using quicklime (CaO) or limestone (CaCO 3) during the iron and steel making processes Quicklime is used in the hot metal Natural and enhanced carbonation of lime in its different 2022年10月1日 However, the calcium looping design benefits from high grade waste heat used in a steam cycle, offsetting the higher CAPEX and greater consumption of fossil fuel It is Decarbonising the lime industry: Stateoftheart

Direct Capture of CO2 from Ambient Air
2016年8月25日 Carbonation–calcination uses the carbonation reaction between quicklime (calcium oxide, CaO) and CO 2 to produce limestone (calcium carbonate, CaCO 3) Calcium carbonate is then introduced in a calcination 2015年4月1日 With the byproduct calcium carbonate is recalcined in a lime kiln to recover lime for reuse (Shen et al, 2011) High purity lime is used in making Precipitated Calcium Lime in the limelight ScienceDirect2019年10月14日 During this process, sodium hydroxide is converted to sodium carbonate The pulp mill then adds calcium oxide, also known as quicklime, to convert the sodium carbonate Applications of Quicklime Hydrated Lime blitzco2021年3月3日 Quicklime is obtained by calcination of calcium carbonate (CaCO 3) to less than its melting point, resulting in the dissociation of calcium carbonate into calcium oxide (CaO) Lime SpringerLink
.jpg)
Investigating the Kinetics, Mechanism, and Activation Energy
2020年9月15日 Quicklime is highly reactive to water and even reacts with moisture in the air Therefore, it is rarely found naturally in nature, and its production in most cases requires 2020年11月29日 Calcination of limestone at high temperature to decompose it to CaO (quick lime) and CO 2 Hydrated or slaked lime is produced by reaction of quick lime with water Refractories for Lime Calcination SpringerLinkJ Phys Chem C 114(30):12948–12954 Chakraborty D, Agarwal V, Bhatia S, Bellare J (1994) Steadystate transitions and polymorph transformations in continuous precipitation of calcium carbonate Ind Eng Chem Res 33(9):2187–2197 Chan CM, Wu J, Li JX, Cheung YK (2002) Polypropylene/calcium carbonate nanocompositesSynthesis of precipitated calcium carbonate: a review2021年1月1日 Lime is a product derived from the thermal decomposition of limestone (mainly calcium carbonate, CaCO3) into quicklime (CaO) and carbon dioxide (CO2), also called calcination(PDF) Natural and enhanced carbonation of lime

Quicklime Formula, Uses, Definition Britannica
2024年11月8日 quicklime (CaO), compound of one atom of calcium and one atom of oxygen that is a white or grayish white solid produced in large quantities by roasting calcium carbonate so as to drive off carbon dioxideAt room 2024年9月23日 material containing calcium carbonate to a temperature of around 1000°C for several hours In this process, known as 'calcining' or simply 'burning', the carbon dioxide in the calcium carbonate is driven off leaving calcium oxide plus any impurities Quicklime is a chemically unstable and hazardous material and is therefore normallyLime An IntroductionCalcium supplements may increase the incidence of constipation, severe diarrhea, and abdominal pain 1, 62 It highlights that calcium carbonate is more often associated with gastrointestinal side effects, including constipation, flatulence, and bloating 18 Of the 92,000 adverse events recorded, the occurrence of constipation was increased with The good, the bad, and the ugly of calcium supplementation: Calcium Oxide, also known as quicklime, lime water, or burnt lime, is a chemical compound with the formula CaO Learn about the preparation, properties, and uses of Calcium oxide here Calcium carbonate undergoes calcination at temperatures ranging between 1070 o C1270 o C These reactions are usually held in a rotary kilnCalcium Oxide (CaO) Preparation, Properties Uses of Quicklime
.jpg)
limestone, quicklime and slaked lime chemguide
2020年12月22日 Calcium oxide is traditionally known as quicklime If you add water to calcium oxide, you get calcium hydroxide (slaked lime) CaO(s) + H 2 O(l) Ca(OH) 2 (s) There is a useful bit of video which shows the conversion of calcium 2024年9月5日 Furthermore, quicklime finds applications in the production of other chemicals, such as calcium carbonate (CaCO 3) and calcium chloride (CaCl 2) These compounds are used in industries such as manufacturing, construction, and water treatment The versatility of quicklime in chemical processes makes it an essential component in numerous Uses Of QuicklimeCharacterization of direct reduced iron for reduction and elimination of sulfur using calcium carbonate Journal of the Korean Ceramic Society 2020 , 57 (1) , 106111Reaction between calcium carbonate and sulfur dioxide2014年6月20日 dry form or in flue gas desulphurization in slurry form The use of lime in carbonate form is beyond the scope of this paper We will concentrate on lime as calcium oxide and calcium hydroxide In most pollution control applications lime is used as calcium hydroxide To manufacture calcium hydroxide the limestone calcium carbonate must be An Overview of Lime Slaking and Factors That Affect the

Lime SpringerLink
2021年3月3日 Lime is the least expensive and one of the most heavily used alkali in the world, being essential to our society It is a nonhydraulic binder, excepting the socalled hydraulic lime, meaning that it will not set under waterFor this reason, lime is sometimes called air lime since it hardens on exposure to air It was used for thousands of years in masonry mortars to bind the 2024年10月26日 Hydrated lime, also known as calcium hydroxide or slaked lime, is made by mixing quicklime (calcium oxide) with water The resulting material is a dry white powder Agricultural lime contains calcium carbonate Using Hydrated Lime in the Garden Safely and 2019年3月20日 Lime is a product derived from limestone in an industrial process Naturally occurring limestone, which is composed almost exclusively of calcium carbonate, transforms into quicklime, calcium oxide, by applying heat When slaked with water, quicklime transforms into hydrated lime, which is a dry powder composed of calcium hydroxideWE LOVE LIME AND ITS BENEFITS! CarmeuseCalcium Oxide Quicklime Calcium oxide (chemical formula: CaO), also called quicklime or burnt lime, is a widely used chemical compound in our daily lives formed by ionic bonding between one calcium atom and one oxygen atom The white or grayishwhite crystalline solid, calcium oxide can be produced in large quantities by driving off carbon dioxide from calcium carbonateCalcium Oxide Quicklime Chemical Formula, Uses
.jpg)
Quick Lime Preparation, Properties and Uses Hebei
2023年10月11日 Quicklime, also referred to as lime (calcium oxide (CaO)), is derived from high quality, natural deposits of limestone (calcium carbonate (CaCO3)) or dolomitic limestone (calcium magnesium carbonate (CaCO3 + MgCO3) Quicklime is produced by heating the stone to almost 2000 degrees Fahrenheit2023年1月12日 The calcium carbonate resulting from this reaction will settle out of the water The sodium sulfate is not a hardnesscausing compound, so it can remain in the water without causing problems quicklime (CaO), also known as calcium oxide or unslaked lime, Caustic soda has the advantages of stability in storage, lower sludge formation Lesson 18: Softening Mountain Empire Community College2020年6月8日 During this process, sodium hydroxide is converted to sodium carbonate The pulp mill then adds calcium oxide, also known as quicklime, to convert the sodium carbonate back to sodium hydroxide in order to use it again The resulting reaction produces a very fine precipitated calcium carbonate (CaCO3) This filler is used in paper production to Application of Quick Lime Hydrated Lime blitzco2019年10月14日 During this process, sodium hydroxide is converted to sodium carbonate The pulp mill then adds calcium oxide, also known as quicklime, to convert the sodium carbonate back to sodium hydroxide in order to use it again The resulting reaction produces a very fine precipitated calcium carbonate (CaCO3) This filler is used in paper production to Applications of Quicklime Hydrated Lime blitzco

Applications of Quicklime Hydrated Lime blitzco
2019年12月7日 1 Calcium carbonate Limestone is a naturally occurring mineral it is essentially calcite, which is theoretically composed of calcium carbonate Earth’s crust contains more than 4 % of calcium carbonateIt is a chemical compound with the formula CaCO 3 and a common substance found in rocks as the minerals calcite andQuicklime is a calcium oxide formed to release carbon dioxide by calcinating calcium carbonate (limestone) Quicklime is also referred to as handpicked lime, burnt lime, lump lime, calcining lime, and caustic lime It is known to be a caustic material that is prepared at approximately 900 degrees Celsius by burning calcium carbonate limestone Quicklime Preparation, Properties, and Applications with 2023年12月21日 Calcareous soils are characterized by the presence of calcium carbonate (CaCO3) in the parent material and lime deposition These soils are generally known to have a pH 7 and above(PDF) The Advantages and Disadvantages of Calcareous Soils vvEPA United States Environmental Protection Agency Office of Water Washington, DC EPA832F00018 September 2000 Waste water Technology Fact Sheet Chemical Precipitation DESCRIPTION Chemical precipitation is a widely used, proven technology for the removal of metals and other inorganics, suspended solids, fats, oils, greases, and some other organic Wastewater Technology Fact Sheet Chemical Precipitation
.jpg)
SelfHealing Concrete as a Prospective Construction
In this review study, an overview of selfhealing techniques based on microbiological calcium carbonate production is presented Furthermore, numerous obstacles associated with crack treatment by microbiological factors are explored, as well as providing recommendations for future study areas quicklime: 3% of cement : Water: Mechanical Fig 4 TEM images of PCC nanoparticles obtained at t = 20 °C and κ 25 = 70 mS cm1: (a) in the absence of NaS and (b) in the presence of NaS at m NaS/m CaO = 002 Fig 5 TEM images of PCC INFLUENCE OF SODIUM STEARATE ON THE 1 天前 The use of lime and soda ash permits hardness reduction down to 05 gr/gal, or about 8 ppm, as calcium carbonate Magnesium is reduced to 25 ppm because of the lower solubility of magnesium hydroxide at the elevated temperatures Today, continuous sludgecontact softeners (see Figures 74 and 75) are used to provide a constant flow with Water Handbook Precipitation Softening Veolia2017年3月3日 Results ensured that the scales formation of calcium carbonate, calcium sulfate, barium sulfate and strontium sulfate, and their relative quantities have been functions of pressure, temperature Beneficiation and Mineral Processing of Calcium Carbonate and Calcium
.jpg)
10 Calcium Carbonate Uses Benefits + Side Effects
2021年9月9日 Calcium carbonate is found throughout the world The forces of nature produce it from the sediment of shells and other fossils over millions of years, the most famous example being limestone It also builds seashells and eggshells The average eggshell contains about 22 g of calcium carbonate [1, 2]! Calcium carbonate is made of calcium 2017年1月1日 PDF Calcium carbonate (CaCO3) is the most widely used filler material in paper, paint, plastic, food, ceramic, cosmetic, medicine and other Find, read and cite all the research you need on Precipitated Calcium carbonate production, synthesis and properties2022年11月28日 What are the advantages and disadvantages of softening? Reading Assignment Read the online lecture Measurements of hardness are given in terms of the calcium carbonate equivalent quicklime (CaO), also known as calcium oxide or unslaked lime, must be slaked before it is used The lime is fed with a dry chemical feeder and mixed with Lime Softening Mountain Empire Community CollegeJ Phys Chem C 114(30):12948–12954 Chakraborty D, Agarwal V, Bhatia S, Bellare J (1994) Steadystate transitions and polymorph transformations in continuous precipitation of calcium carbonate Ind Eng Chem Res 33(9):2187–2197 Chan CM, Wu J, Li JX, Cheung YK (2002) Polypropylene/calcium carbonate nanocompositesSynthesis of precipitated calcium carbonate: a review
.jpg)
(PDF) Natural and enhanced carbonation of lime
2021年1月1日 Lime is a product derived from the thermal decomposition of limestone (mainly calcium carbonate, CaCO3) into quicklime (CaO) and carbon dioxide (CO2), also called calcination2024年11月8日 quicklime (CaO), compound of one atom of calcium and one atom of oxygen that is a white or grayish white solid produced in large quantities by roasting calcium carbonate so as to drive off carbon dioxideAt room Quicklime Formula, Uses, Definition Britannica2024年9月23日 material containing calcium carbonate to a temperature of around 1000°C for several hours In this process, known as 'calcining' or simply 'burning', the carbon dioxide in the calcium carbonate is driven off leaving calcium oxide plus any impurities Quicklime is a chemically unstable and hazardous material and is therefore normallyLime An IntroductionCalcium supplements may increase the incidence of constipation, severe diarrhea, and abdominal pain 1, 62 It highlights that calcium carbonate is more often associated with gastrointestinal side effects, including constipation, flatulence, and bloating 18 Of the 92,000 adverse events recorded, the occurrence of constipation was increased with The good, the bad, and the ugly of calcium supplementation:

Calcium Oxide (CaO) Preparation, Properties Uses of Quicklime
Calcium Oxide, also known as quicklime, lime water, or burnt lime, is a chemical compound with the formula CaO Learn about the preparation, properties, and uses of Calcium oxide here Calcium carbonate undergoes calcination at temperatures ranging between 1070 o C1270 o C These reactions are usually held in a rotary kiln2020年12月22日 Calcium oxide is traditionally known as quicklime If you add water to calcium oxide, you get calcium hydroxide (slaked lime) CaO(s) + H 2 O(l) Ca(OH) 2 (s) There is a useful bit of video which shows the conversion of calcium limestone, quicklime and slaked lime chemguide2024年9月5日 Furthermore, quicklime finds applications in the production of other chemicals, such as calcium carbonate (CaCO 3) and calcium chloride (CaCl 2) These compounds are used in industries such as manufacturing, construction, and water treatment The versatility of quicklime in chemical processes makes it an essential component in numerous Uses Of QuicklimeCharacterization of direct reduced iron for reduction and elimination of sulfur using calcium carbonate Journal of the Korean Ceramic Society 2020 , 57 (1) , 106111Reaction between calcium carbonate and sulfur dioxide
.jpg)
An Overview of Lime Slaking and Factors That Affect the
2014年6月20日 dry form or in flue gas desulphurization in slurry form The use of lime in carbonate form is beyond the scope of this paper We will concentrate on lime as calcium oxide and calcium hydroxide In most pollution control applications lime is used as calcium hydroxide To manufacture calcium hydroxide the limestone calcium carbonate must be