How to convert 5 calcium carbonate high calcium stone mill base to 10
.jpg)
physical chemistry Purity of calcium carbonate in limestone
2016年1月4日 $\mathrm{150~mL}$ of $\mathrm{01~N}$ $\ce{HCl}$ is required to react with $\mathrm{1~g}$ of a sample of limestone Calculate percentage purity of calcium carbonate How to Calculate Limestone Effective Calcium Carbonate Equivalent (ECCE) % of Particles Fineness Percent Available Passing Each Screen Factor Based on Fineness 4mesh 100 x Soil pH and LimingThis conversion of calcium carbonate to calcium oxide is achieved by heating the limestone to a temperature high enough (eg 1000 C in a lime kiln) to 'drive off' carbon dioxide, CO 2 The HOW TO CALCULATE EFFICIENCY OF YOUR LIME BURNING The carbon dioxide reacts with the calcium hydroxide to form white calcium carbonate, which is insoluble and so turns the limewater ‘milky’ calcium hydroxide + carbon dioxide → calciumLimestone [GCSE Chemistry only] The limestone cycle
.jpg)
Soil Acidity and Liming: Basic Information for Farmers
2 天之前 There is no minimum calcium carbonate equivalent requirement for limestone sold in North Carolina However, the product must be labeled to 2017年1月3日 Calcium oxide is usually made by the thermal decomposition of materials, such as limestone or seashells, that contain calcium carbonate ($\ce{CaCO3}$; mineral calcite) in a How is the process of converting calcium carbonate (CaCO3) into An important and growing use for lime is in the production of precipitated calcium carbonate (PCC), which is used in the production of paper, paint, ink, plastic, and rubber The paper Limestone and Crushed Rock Department of EnergyCalcium carbonate is a chemical compound with the chemical formula Ca CO 3 It is a common substance found in rocks as the minerals calcite and aragonite, most notably in chalk and Calcium carbonate Wikipedia

The Transformation Process within a Lime Kiln: Breaking It Down
At its core, the primary function of a lime kiln is to convert limestone (calcium carbonate) into quicklime (calcium oxide) through a process called calcination This process is accomplished If you add water to calcium oxide, you get calcium hydroxide (slaked lime) CaO(s) + H 2 O(l) Ca(OH) 2 (s) There is a useful bit of video which shows the conversion of calcium carbonate limestone, quicklime and slaked lime chemguideThis conversion of calcium carbonate to calcium oxide is achieved by heating the limestone to a temperature high enough (eg 1000 C in a lime kiln) to 'drive off' carbon dioxide, CO 2 The equation for this process, with the approximate molecular weights, is: 100 CaCO 3 + Heat 56 CaO + 44 CO 2 Continuing the approximation for hydration gives:HOW TO CALCULATE EFFICIENCY OF YOUR LIME BURNING Calcium Carbonate Formula It is a chemical compound with the chemical formula CaCO 3; It is a white insoluble powderlike substance which occurs naturally in minerals, chalk, marble, limestone, calcite, shells, pearl, etc; Medicinally, it is Limestone: Calcium Carbonate (CaCO3) Uses,
Calcium Carbonate (CaCO3) Structure, Properties, Uses of Calcium
Calcium Carbonate (CaCO3) Calcium carbonate is the salt of a strong base High calcium levels can also cause serious heart rhythm disturbances Q5 What are the benefits of calcium tablets? Calcium is key to growing new bone and keeping the bone you have strong2023年8月5日 Calcium carbonate is an inorganic salt primarily used to manage and treat low calcium conditions, GERD, CKD, and other indicated conditions Calcium carbonate is classified as a calcium supplement, antacid, and phosphate binder This activity outlines the significant indications, actions, and contraindications for calcium carbonate as a valuable agent in Calcium Carbonate StatPearls NCBI Bookshelf2023年6月25日 Crushing: The calcium carbonate stones just mined from the quarry are relatively large, and they need to be crushed by a jaw crusher and a hammer crusher in turn to the feed fineness (10mm20mm) that can enter the mill Grinding: Use a bucket elevator to send the crushed small pieces of calcium carbonate to the silo, then use a vibrating feeder to send them Guide to Calcium Carbonate Grinding: Mills, Tips, and Uses2021年7月8日 Meals: Different types of calcium vary in whether they're absorbed best with or without foodCalcium carbonate should be taken with meals Calcium citrate should be taken on an empty stomach Medications: Calcium should not be taken with certain medications, including antibiotics, iron supplements, high blood pressure medications, and othersCalcium Citrate vs Calcium Carbonate: Which to Take? Verywell
)D`ERF`389RMI4.jpg)
How To Calculate Alkalinity As Concentration Of CaCO3
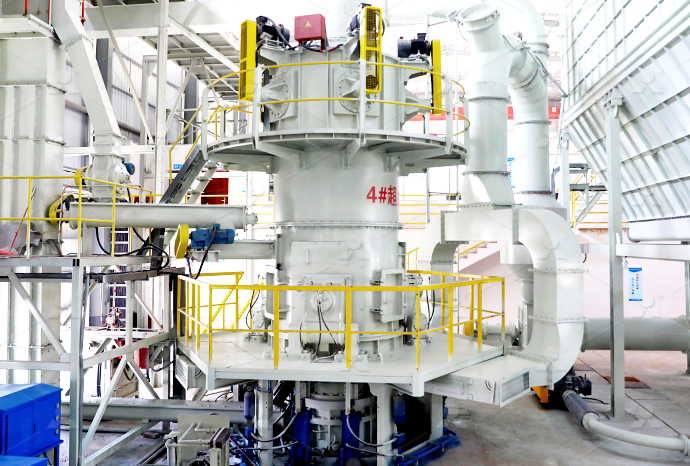
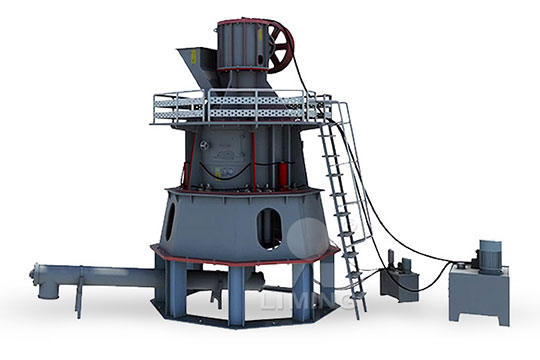
2018年6月4日 TL;DR (Too Long; Didn't Read) Use this equation to calculate the alkalinity in terms of CaCO 3: Alk(mg/L CaCO 3)=Alk(meq/L)x(1mmol CaCO 3 ÷2meq)x(100087mg CaCO 3 ÷1mmol CaCO 3)=(50044xBxC a xCF)÷V sThe variables used are the volume of acid (B) used in the titration, the concentration of the acid (C a), the volume of water in the sample (V s) and a 2023年9月19日 Project Background The calcium carbonate industry has been developing in my country for many years and has accumulated rich experience and production technology has also been significantly improved Calcium carbonate is usually used as a filler, and is also widely used in daily chemical industries such as artificial floor tiles, coatings, paints, inks, cables, rubber, Calcium carbonate milling process SBM Ultrafine Powder Calcium carbonate ultrafine vertical mill, mainly used for the production of calcium carbonate powder Calcium carbonate powder can be widely used in cables, papermaking, toothpaste, cosmetics, glass, medicine, paint, ink and many other Calcium Carbonate Ultrafine Vertical Mill SBM Ultrafine 2023年11月14日 Calcium carbonate supplements are an effective way to increase your calcium intake if your diet isn’t sufficient, or you have a condition that leads to lower calcium levels Learn about Calcium Carbonate: Uses, Dosage, and Potential Side Effects

Frontiers Calcium Carbonate Precipitation for CO2
2017年7月9日 In general, calcium carbonates exist either in the form of amorphous calcium carbonate (ACC) or one of the three polymorphs, namely, calcite, aragonite, and vaterite (Figure 1) Two hydrated phases of calcium 2016年1月8日 When calcium carbonate crystallizes into hard shells, it incorporates soft bits of proteins to add strength Research done in the lab shows how this might happen, and why it worksStudy shows how calcium carbonate forms 2017年1月1日 PDF Calcium carbonate (CaCO3) is the most widely used filler material in paper, paint, plastic, food, ceramic, cosmetic, medicine and other Find, read and cite all the research you need on Precipitated Calcium carbonate production, Contraindicated (1) ceftriaxone ceftriaxone, calcium carbonate Other (see comment) Contraindicated Comment: Do not use ANY calcium containing solutions (including Ringer or Harmann solutions) in combination with IV ceftriaxone; risk of potentially fatal particulate precipitation in lungs, kidneysTums (calcium carbonate) dosing, indications, interactions,
.jpg)
Convert grams Calcium Carbonate to moles Conversion of
More information from the unit converter How many grams Calcium Carbonate in 1 mol? The answer is 1000869 We assume you are converting between grams Calcium Carbonate and moleYou can view more details on each measurement unit: molecular weight of Calcium Carbonate or mol The molecular formula for Calcium Carbonate is CaCO3The SI base unit for 2021年1月11日 Eggshells are made primarily of calcium carbonate (CaCO 3), plus small amounts of calcium phosphate and magnesium carbonate Calcium carbonate is insoluble in water, which plant roots use to uptake nutrients White vinegar contains about 3% acetic acid When the acetic acid reacts with the calcium carbonate, you’re left with watersoluble How to Make Water Soluble Calcium Eggshell Fertilizer2023年9月6日 3 Marble and Other CalciumBased Stone: For marble and other calciumbased stone surfaces, it is important to use cleaners that are both stonesafe and pHneutral For polished stone, there are specific products available specifically designed for this type of surface These will help prevent any streaks or residue from appearing after cleaningHow To Clean Calcium Buildup on Shower Floor 5 DIY Methods2022年9月27日 The energy band gap of CaCO3 sample from chicken eggshell synthesis Figure 3 shows the absorption rate of CaCO 3 from the synthesis of chicken egg shells in the range of 2371 nm, 2515 nm, and (PDF) SYNTHESIS OF CALCIUM CARBONATE (CaCO3) FROM

Calcium carbonate Formula, Uses, Names, Facts Britannica
2024年10月26日 As marble, calcium carbonate is used for statuary and carvings and is a popular facing stone as polished slabsThe term marble is used differently in the marketplace from the way it is used in geology: in the marketplace, it is applied to any coarsegrained carbonate rock that will take a good polish rather than to metamorphic carbonaterich rocks exclusively2017年1月31日 The aqueous sodium carbonate is subsequently treated with calcium hydroxide to precipitate out calcium carbonate: $$\ce{Na2CO3 (aq) + Ca(OH)2 > 2NaOH (aq) + CaCO3 (s)}$$ Disclaimer: This is usually done with much higher concentrations of $\ce{CO2}$ (eginorganic chemistry How to convert Sodium Carbonate to 2020年12月20日 Calcium Carbonate (CaCO3), a fundamental inorganic material, has been played an important role as fillers in paper making plastics, rubbers and coating because of its reasonable price and (PDF) SYNTHESIS OF CALCIUM CARBONATE 2018年2月25日 Robert makes an excellent point here! Just try soaking a hard boiled egg in vinegar overnight This will extract all of the calcium from the shell (1 shell is about 2000mg calcium) The vinegar causes the carbonate to be How to Make Eggshell Calcium (and Why You’d Want
.jpg)
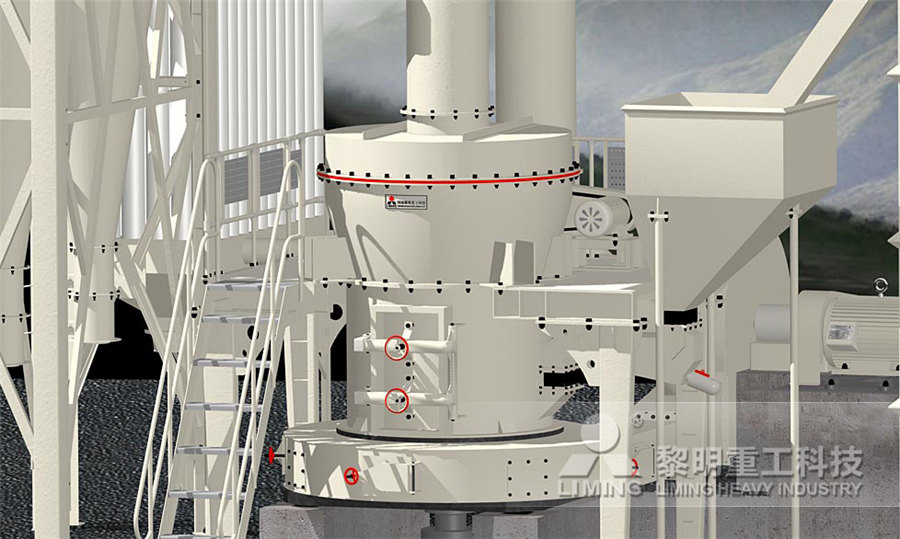
calcium carbonate (CaCO 3 ) equivalent California State
calcium carbonate (CaCO 3) equivalent An expression of the concentration of specified constituents in water in terms of their equivalent value to calcium carbonate For example, the hardness in water that is caused by calcium, magnesium, and other ions is usually described as calcium carbonate equivalentTechnical Advantages 1 Higher yield and better quality In order to avoid the problems of long material residence time, repeated grinding, and high iron content in the grinding process of traditional mills, SBM specially designed a unique grinding curve for the roller shell and linerCalcium Carbonate Ultrafine Powder Vertical MillFringing reefs off Stone Town (Figure 1C), located in the western shoreline of Zanzibar Island, are exposed to sewage outfall waters that discharge into coastal watersThe main outfall, located adjacent to the Port of Stone Town, discharges an estimated 22 × 10 6 L d −1 of untreated sewage (Moynihan et al, 2012)Tidal currents distribute the flow of wastewater in a northward Calcium Carbonate Production, Coral Cover and Diversity along a When calcium carbonate is heated strongly, it decomposes to give calcium oxide and carbon dioxide CaCO 3 (s) CaO(s) + CO 2 (g) Calcium oxide is traditionally known as quicklime If you add water to calcium oxide, you get calcium hydroxide (slaked lime) limestone, quicklime and slaked lime chemguide
.jpg)
Extraction of iron IGCSE Chemistry Revision Notes Save My Exams
2024年11月4日 The calcium carbonate in the limestone thermally decomposes to form calcium oxide calcium carbonate → calcium oxide + carbon dioxide The calcium oxide formed reacts with the silicon dioxide, which is an impurity in the iron ore, to form calcium silicate This melts and collects as a molten slag floating on top of the molten iron, which is Base saturation Base saturation is the percentage of the soil CEC occupied by basic cations (calcium, magnesium, potassium, sodium) at the current soil pH value Base saturation and CEC are nearly equivalent (base saturation is 100%) when soil pH is near 7 An acidic soil has a base saturation of about 50% when the soil pH is about 5Soil Test Interpretation Guide OSU Extension ServiceIdentification of carbonate rocks Limestone is easily recognizable because it fizzes in contact with HCl (hydrochloric acid), due to the reaction: CaCO 3 + 2HCl ⇌ CO 2 + H 2 O + CaCl 2 Dolostone does not fizz on a 10% diluted HCl solution, but can fizz if the solution is put in contact with a dolomite powder or if a less diluted solution (around 30%) is usedCarbonate Rocks Geology is the WayWhether the aquarium water is naturally high in KH or if the pH level of the aquarium is high, then it is absolutely necessary to lower the KH While it is easy to increase the KH, lowering it is a bit complex You will have to strike a balance between pH and KH to prevent any swings in pH level, which could prove fatal for your fishHow to Lower KH (Carbonate Hardness) in an Aquarium?
.jpg)
67: Acidbase reactions Chemistry LibreTexts
2023年4月3日 Reactions of acids with carbonates and hydrogen carbonates Carbonic acid (H 2 CO 3) is a weak acid found in carbonated waterThe H 2 CO 3 is a product of carbon dioxide (CO 2) and water (H 2 O) by the following 2020年7月30日 In this study, to determine the calcium carbonate availability in eggshells waste and factors those affect its extraction The parameters like temperature, the size of the eggshell powder and the (PDF) Calcium Carbonate Synthesis, Optimization The primary source of alkalinity is carbonatecontaining rocks, which can come from: • Natural erosion of carbonatecontaining “limestone,” such as calcium carbonate or dolomite; • Runoff from agricultural or other landscapes where “lime” has been Alkalinity Kennesaw State University2016年3月1日 Biomineralization is a naturally occurring process in living organisms In this review, we discuss microbially induced calcium carbonate precipitation (MICP) in detail In the MICP process, urease plays a major role in urea hydrolysis by a wide variety of microorganisms capable of producing high levels of urease We also elaborate on the different polymorphs and Formations of calcium carbonate minerals by bacteria and its

drinking Water treatment: carbonate removal and/or softening
Sections removing hardness (calcium and magnesium) and classic schemes used describe the processes used to correct water that is too hard by using the chemical precipitation (carbonate removal) or ion exchange methods, respectively softening through carbonate removal When the water has a high TH accompanied by a significant Malk, the water can be softened by using There is a solid mixture which include Calcium carbonate and has other inert stuff 55 g of that mixture is mixed with 10 cm 3 of 10 mol dm3 HCl solution After allowing reaction to be completed, pH value of the aqueous solution is measured and pH value was 3 Calculate the composition of Calcium carbonate in weight basis (m/m%)Calcium Carbonate and Hydrochloric Acid Reaction CaCO32024年4月16日 Find a source of calcium carbonate Your first step is going to be finding the raw material for quicklime These raw materials can be purchased at garden stores, hardware stores, or construction material suppliers The main source of lime is rock that contains calcium carbonate In picking a source of lime, consider: Dolomite; Chalk; LimestoneHow to Make Quicklime: 10 Steps (with Pictures) wikiHowIf 14 g of calcium oxide is formed by the complete decomposition of calcium carbonate, then the amount of calcium carbonate taken and the amount of carbon dioxide formed will be respectively : (a) 22 g and 11 g (b) 11 g and 25 g (c) 25 g and 11 g (d) 50 g and 11 gThe conversion of calcium carbonate into calcium oxide is an

Calcium intake and absorption Mayo Clinic Health System
2024年2月29日 Calcium is the most abundant mineral in the body About 1% of the body's calcium is used for metabolic functions, such as vascular contraction and dilation, muscle function, blood clotting, heart rhythm, nerve transmission, intracellular signaling and hormone secretionThe remaining 99% is found in structural support for your bones and teethAbstract Calcium (Ca +2) is a divalent cation that plays a critical role in numerous body functions such as skeletal mineralization, signal transduction, nerve conduction, muscle contraction, and blood coagulationCa +2 metabolism is linked to magnesium (Mg +2) and phosphate metabolismCa +2 homeostasis is dependent on intestinal absorption, bone turnover, and Disorders of Calcium Metabolism: Hypocalcemia and