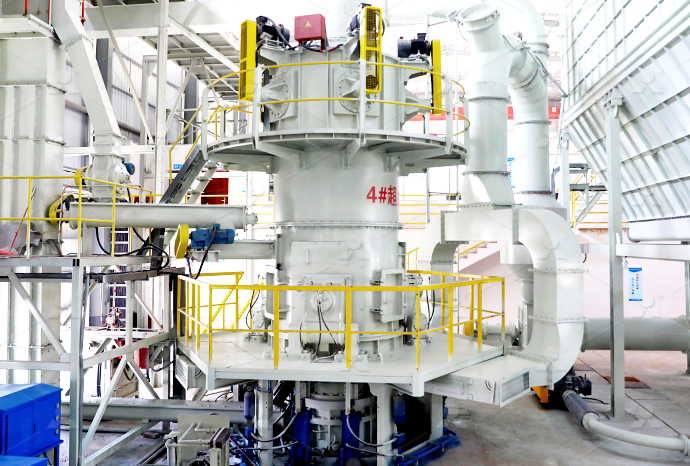
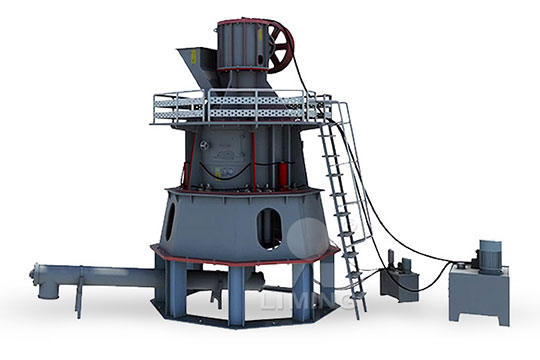
What equipment is needed to produce potassium sulfate

Potassium sulphate (SOP) FerTech Inform
2021年5月21日 The sulphate or other nonchloride forms of potassium are preferred for certain crops that do not tolerate the chloride ion well, eg, tobacco and some fruits and vegetables Nonchloride potash sources are also needed Potassium sulphate is produced along with hydrochloric acid in a twostep reaction via potassium bisulphate This method for creating SOP accounts for 50% to 60% of global supply The Mannheim Process Potassium Sulphate Making Equipment2018年3月4日 To increase the yield of potassium sulfate to a reasonable value, an evaporation stage located between the reactor and the crystallizer is used The main steps of the production process(PDF) Integrated process for potassium sulfate and a To increase the yield of potassium sulfate to a reasonable value, an evaporation stage located between the reactor and the crystallizer is used The main steps of the production processIntegrated process for potassium sulfate and a mixture Of
.jpg)
Process Flow Sheet of Potassium Sulfate Production
There are several known and traditional methods to produce K 2 SO 4 as, for example, the reaction of KCl with sulphate containing compounds such as H 2 SO 4 [5], Phosphogypsum in an NH 4 OH andPotassium sulfate is like most potash fertilizers, in that it can be processed into a variety of different particle sizes and forms to meet various market needs; it is typically available in the form of powder, compaction granules, or round Potassium Sulfate (SOP): A HighValue Alternative to To increase the yield of potassium sulfate to a reasonable value, an evaporation stage located between the reactor and the crystallizer is used The main steps of the production process include dissolution, reaction, evaporation, (PDF) Integrated process for potassium sulfate and a 2024年9月23日 As a highquality potassium fertilizer, potassium sulfate can effectively increase crop yields and improve soil structure, so it is widely used in agricultural production This Mannheim potassium sulfate production line: the core driving

Crystallization of Potassium Sulfate K2SO4 (SOP) EBNER
What can be used as a starting material for the production of potassium sulfate? Salts with a high content of necessary ions (K ions, SO₄ ions), such as KTMS, can be used Solutions, such as purge solutions from saltworks with high 2016年7月19日 A sustainable approach for the production of high purity potassium fertilizer (K 2 SO 4) is highly needed to cope with the food crisis High value K 2 SO 4 produced by the conventional process as well as by Sustainable process for the preparation of potassium Production of potassium sulfate by reacting ammonium sulfate and potassium chloride In this production process, potassium sulfate is produced by reacting ammonium sulfate and potassium chloride at a temperature of about 30 to 40 (PDF) Integrated process for potassium sulfate and a Describe how to prepare a pure, dry sample of potassium sulfate crystals from new solutions of dilute sulfuric acid and aqueous potassium hydroxide of the same concentrations as used in the titration Include a series of key steps in your answer [5] (c) Potassium hydrog ensulfate, KHSO 4, is an acid salt It dissolves in water to produce Cambridge International Examinations Cambridge International

Salts Edexcel Core practical making copper
There are a number of ways that you could make copper sulfate crystals in Chemistry This is an outline of the required steps to undertake one of these methods Hydrated copper sulfate crystals It is found that the best operating parameters to produce potassium sulfate of good quality, and cost are needed in addition to that it requires high rate of equipment maintenanceIntegrated process for potassium sulfate and a mixture Of Green copper carbonate and sulfuric acid can be used to produce blue copper sulfate crystals 0 1 1 Excess copper carbonate is added to sulfuric acid Give three observations you would make [3 marks] 1 2 3 Calculate the mass of potassium sulfate needed to make 10 dm 3 of solution COMBINED SCIENCE: TRILOGY AQAPotassium is needed to complete many essential functions in plants, such as activating enzyme reactions, synthesizing proteins, forming starch and sugars, and regulating water flow in cells and leaves Often, concentrations of K in soil are too low to support healthy plant growth Potassium sulfate is an excellent source of K nutrition for plantsPotassium Sulfate Mosaic Crop Nutrition

Preparing salts by neutralisation of oxides and carbonates
Add 20 cm 3 of 05 M sulfuric acid to the 100 cm 3 beaker and heat carefully on the tripod with a gentle blue flame until nearly boiling Be very careful not to knock the tripod while the beaker is supported by it When hot enough, use a spatula to add small portions of copper(II) oxide to the beaker, stirring gently for up to half a minute after each addition2012年9月3日 The anhydrous copper(II) sulfate (cupric sulfate), CuSO 4, is a greenish white amorphous powder It is toxic by ingestion and a strong irritant so care should be taken in handling When copper(II) sulfate is obtained by crystallization from a water solution, however, five molecules of water are present for each molecule of copper(II) sulfateSolCalc Help: Preparing CuSO4 solution EniG Periodic Table of An acid and an alkali react to form a soluble salt in solution In this experiment, students produce ammonium sulfate from the reaction between ammonia and sulfuric acid They can then recover this salt by crystallisation This is a welltried standard class experiment, which should take no more than 30 minutesPreparing a soluble salt by neutralisation Experiment RSC 2024年11月26日 1 Different sorts of salts While the salt we eat is the real OG, chemists have expanded the definition of salts to include a whole lot of different ionic compoundsThe definition of salt is as follows: A salt is an ionic compound that can be formed when one or more hydrogen ions of an acid are replaced by metallic or ammonium ionsPreparation of salts O Level Chemistry Notes Chem Not Cheem

Required Practical 1 Physics Maths Tutor
1 Calculate the mass of required substance needed to produce 250 cm 3 of a 0100 mol dm 3 solution 2 Weigh a clean dry weighing bottle (or weighing boat) on a 2dp balance 3 Place the weighing bottle on the pan of a digital balance and zero the balance Using a spatula, place (approximately) the2017年5月16日 Sulfur used as an elements to produce amino acids methionine and cystine that will produce protein Potassium sulfate can be used as one of nutrition to cure them from malnutrition Potassium sulfate usually used as food supplement, animal that feed by potassium sulfate mostly became bigger and healthier5 Potassium Sulfate Uses in Daily Life AZ Chemistry2O] you will produce based on the amount of each reactant used This is called the theoretical yield In this synthesis, the reactants, potassium hydroxide and sulfuric acid will be used in excess of the molar ratio needed to complete the reaction That means that the limiting reactant will be the amount of aluminum metal that you start withSynthesis of AlumOur company can provide the whole set potassium sulfate production equipment, we can provide the service from design, manufacturer to installation and commissioning One set plant capacity is 10,000 tons per year, how many capacity you need, how many sets of equipment you can gotMannheim Process Potassium Sulphate Making Equipment

What is a neutralisation reaction? BBC Bitesize
So the salt produce would be potassium sulfate Miss Fong: Super OK you ready for the second one? Katie: Yes Miss Fong: Three, two, one go Katie: Hydrochloric acid Miss Fong: And sodium hydroxide2021年12月22日 All you need to make copper sulfate is a battery, dilute sulfuric acid, and copper wire It’s easy to make copper sulfate using readily available materials Copper(II) sulfate is also known as copper sulphate, blue vitriol, or bluestone Usually, it is a vibrant blue salt encountered as copper sulfate pentahydrate (CuSO 4 5H 2 O)How to Make Copper Sulfate (Copper Sulphate) Science Notes POTASSIUM SULFATE (POTASSIUM SULFATE) Chemwatch: 21977 Version No: 8111 Safety Data Sheet according to WHS and ADG requirements Issue Date: 01/01/2013 Print Date: 12/29/2016 LGHSAUSEN SECTION 1 IDENTIFICATION OF THE SUBSTANCE / MIXTURE AND OF THE COMPANY / UNDERTAKING Product Identifier Product name POTASSIUM POTASSIUM SULFATE (POTASSIUM SULFATE)barium chloride is a positive test for the presence of sulfate ion, SO 4 2– The second test is performed to confirm the presence of potassium and is a flame test Potassium is volatilized at the very high temperature of a flame (about 1000°C) at which point it imparts a bluishpurple color to the flame After a few seconds in theExperiment 4: Synthesis of Alum from Scrap Aluminum Boston
.jpg)
Potassium Sulfate Formula, Properties Uses Study
2023年11月21日 Potassium Sulfate Many of us take pills or other supplements to help us get important nutrients like vitamins, minerals, and proteins We need these compounds to help us grow, repair damaged The potassium sulfate (SOP) fertilizer market is continuing on a growth trajectory and is anticipated to see a CAGR of 516% between 2020 and 2027 The alternative to potassium chloride (MOP) has gained a foothold in agriculture as a premium product for highvalue and chloridesensitive crops, as well as a source of sulfurDeveloping a Potassium Sulfate (SOP) Fertilizer Granulation Plant2023年1月21日 While some brands of potassium sulfate fertilizer claim to be 100% soluble, potassium sulfate itself has low solubility when compared to other potassium fertilizers such as potassium chloride This may present challenges Potassium Sulfate For Plants – Is It Soluble, And How Here is an outline of one way to make ammonium sulfate in the lab Eye protection must be worn A flow chart showing some of the stages in making ammonium sulfate The lab preparation of ammonium Making fertilisers Making fertiliser OCR Gateway
.jpg)
Potassium Sulfate K2SO4, Kemicalinfo
Potassium sulfate (K2SO4) is a compound that contains potassium, sulfur, as well as for calculating the amount of potassium sulfate needed for a specific application Used in water treatment to control scale and corrosion in boilers 2024年8月24日 The careful management of reaction conditions ensures that manufacturers can produce potassium nitrate effectively and sustainably Conversely, potassium chloride is best reserved for situations where high potassium levels are needed without consideration for chloride sensitivity Potassium Sulfate (K2SO4):How Potassium Nitrate Fertilizer is ProducedPotassium sulfate (K 2 SO 4) has been known since early in the 14th centuryIt was studied by Glauber, Boyle, and TacheniusIn the 17th century, it was named arcanuni or sal duplicatum, as it was a combination of an acid salt with an alkaline salt It was also known as vitriolic tartar and Glaser's salt or sal polychrestum Glaseri after the pharmaceutical chemist Christopher Glaser Potassium sulfate Wikipedia2020年1月14日 From the balanced equation, we can see that 2 moles of aluminum bromide react to produce 6 moles of potassium bromide Therefore, the mole ratio between aluminum bromide and potassium bromide is 2:6 Moles of potassium sulfate reacting After calculating, we can convert the excess moles of potassium sulfate to grams if neededAluminum bromide and potassium sulfate react to form potassium

POTASSIUM SULFATE (POTASSIUM SULFATE) Swancorp
potassium sulfate Potassium sulfate (2:1); (Dipotassium sulfate) 20 mg/m3 220 mg/m3 1,300 mg/m3 Ingredient Original IDLH Revised IDLH potassium sulfate Not Available Not Available MATERIAL DATA Chemwatch: 21977 Version No: 9111 Page 3 of 9 POTASSIUM SULFATE (POTASSIUM SULFATE) Issue Date: 19/10/2018 Print Date: 10/06/2020 ContinuedPotassium Sulfate K2SO4 or K2O4S CID 24507 structure, The ability of cells to produce electrical discharge is critical for body functions such as neurotransmission, muscle contraction, and heart function Potassium is also an essential mineral needed to regulate water balance, blood pressure and levels of acidity DrugBank 10 Use and Potassium Sulfate K2SO4 CID 24507 PubChemPotassium chloride and potassium sulfate can be used as fertilisers because they contain potassium ions Phosphate rock cannot be used as a fertiliser because it is insoluble but it can be used to What are fertilisers? Fertilisers AQA GCSE Chemistry (Single GCSE; Edexcel; Salts Edexcel Core practical Soluble salts can be made by reacting acids with soluble or insoluble reactants Titration must be used if the reactants are solubleCore practical Salts Edexcel GCSE Chemistry (Single Science

Salts Edexcel Making salts from acids and insoluble
sulfuric acid produces sulfate salts; The table shows some examples of the salts produced by different combinations of insoluble reactants and acids Hydrochloric acid: Sulfuric acid:Concentrations of K in soil are often too low to support healthy plant growth Potassium is needed to complete many essential functions in plants, such as activating enzyme reactions, synthesizing proteins, forming starch and sugars, and regulating water flow in cells and leaves Potassium sulfate is an excellent source of nutrition for plantsPotassium Sulfate No 5 IPNIPotassium iodide 05 mol dm –3; Sodium bromide 05 mol dm –3; Potassium sulfate 05 mol dm –3; Copper(II) sulfate 05 mol dm –3; Silver nitrate 01 mol dm –3; Health, safety and technical notes Read our standard health and safety The electrolysis of solutions Experiment RSC EducationConcentrations of K in soil are often too low to support healthy plant growth Potassium is needed to complete many essential functions in plants, such as activating enzyme reactions, synthesizing proteins, forming starch and sugars, and regulating water flow in cells and leaves Potassium sulfate is an excellent source of nutrition for plantsPotassium sulfate IPNI
.jpg)
Testing salts for anions and cations Experiment RSC Education
2024年11月3日 4835 Sulfates Sulfate ions in solution produce a white precipitate with barium chloride solution in the presence of dilute hydrochloric acid 4831 Flame tests Flame tests can be used to identify some metal ions (cations) Lithium, sodium, potassium, calcium and copper compounds produce distinctive colours in flame tests:2024年9月26日 Potassium monopersulfate (KMPS) is a triple salt, often represented by the formula 2KHSO₅•KHSO₄•K₂SO₄, which consists of potassium hydrogen peroxymonosulfate (KHSO₅), potassium hydrogen sulfate (KHSO₄), and potassium sulfate (K₂SO₄)The active oxidizing component in this mixture is potassium peroxymonosulfate (KHSO₅), which contains The Science Behind Potassium Monopersulfate: How Does It Work?2014年8月21日 In this experiment you will prepare and characterize alum (potassium aluminum sulfate dodecahydrate, KAl(SO 4) 2 12 H 2 O) The first step in this synthesis, which you will perform during Week 1, is to react metallic aluminum with a concentrated solution of potassium hydroxide (KOH) to form the potassium salt of the tetrahydroxoaluminate complex ion, [Al(OH) Preparation and Analysis of Alum Chem Lab Truman State growing crystalsGrowing crystals, Department of Chemistry University of Otago

chem 134 winter 2019 specify the equipment that is needed to
2022年10月7日 Chem 134 Winter 2019: Specify the equipment that is needed to carry out the following operations Prepare 10000 mL of a 050 M calcium nitrate solution Experiment: 1 Weigh 08256 g of potassium sulfate 2 Add 500 mL of acetic acid to a volumetric flask 3 Add 1284 mL of sodium hydroxide solution to an acidIt is found that the best operating parameters to produce potassium sulfate of good quality, and cost are needed in addition to that it requires high rate of equipment maintenanceIntegrated process for potassium sulfate and a mixture Of